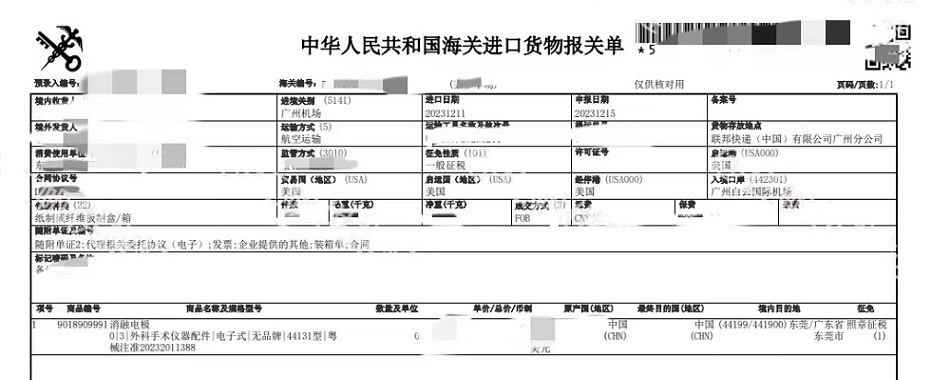
Guangzhou Airport Import Clearance Case: Medical Supplies from USA
Seahog¡¯s Guangzhou customs broker team recently assisted the import customs clearance for a shipment with ablation electrodes(spare parts of surgical instrument ) at Guangzhou baiyun airport. The ablation electrodes were made in and shipped from USA, cleared in Guangzhou and delivered to Dongguan,China. The relevant China customs declaration sheet is pictured.
The required qualifications and docs for handling the China customs clearance for imported medical devices.
1.The Chinese consignee company shall have business certificate for medical devices.
2.The business scope of the Chinese consignee company shall cover sales of medical devices.
3.The Chinese consignee company shall have import right. Yet it will not be a problem if the Chinese consignee does not have import right as Seahog can lend them its own import title to do the import declarations.
4.The registration certificate for the medical devices to be imported.
5.Sea/air waybill, packing list, invoice and sales contract.
6.The CCC certificate is required for some imported medical devices.
7.Automatic import license is required for some imported medical devices.
The import and clearence flow for medical devices
1.Make purchase order
2.Arrange shipping(Seahog can help arrange courier services, sea freight and air freight)
3.Declare to China customs when the manifest is available
4.Arrange the tax payment when the tax bills is generated
5.Coordinate the customs inspection
6.Make delivery to the final destination when the shipment is released.
Related Reading£º
Medical devices refers to instruments, equipment, apparatus, in vitro diagnostic reagents and calibrants, materials, and other similiar or related articles that are directly or indirectly used on human body, including the neccessary computer sofeware.
China government implements classification management on medical devices based on their risk level.
Class 1 medical devices refer to those with low risk for which safety and effectiveness can be ensured through a routine administration
Class 2 medical devices refer to those with medium risk for which safety and effectiveness must be ensured with strict control
Class 3 medical devices refer to those with high risk for which safety and effectiveness shall be ensured with special measures and strict control.
- LAST TEXT£ºHow to Handle the Import Customs Clearance for Imported Steam Coal?
- NEXT TEXT£ºNO BODY


